
PHYSICS
Figure 4
The tap is closed, the tank is under pressure, for example with
p
= 5
bar
. When the tap is opened, the air inside
the tank releases into the atmosphere until the two pressures reach equilibrium. To achieve this equilibrium the
molecules were transferred from inside to outside the tank.
Fig. 3
open
1bar
5bar
closed
1bar
1bar
Fig. 4
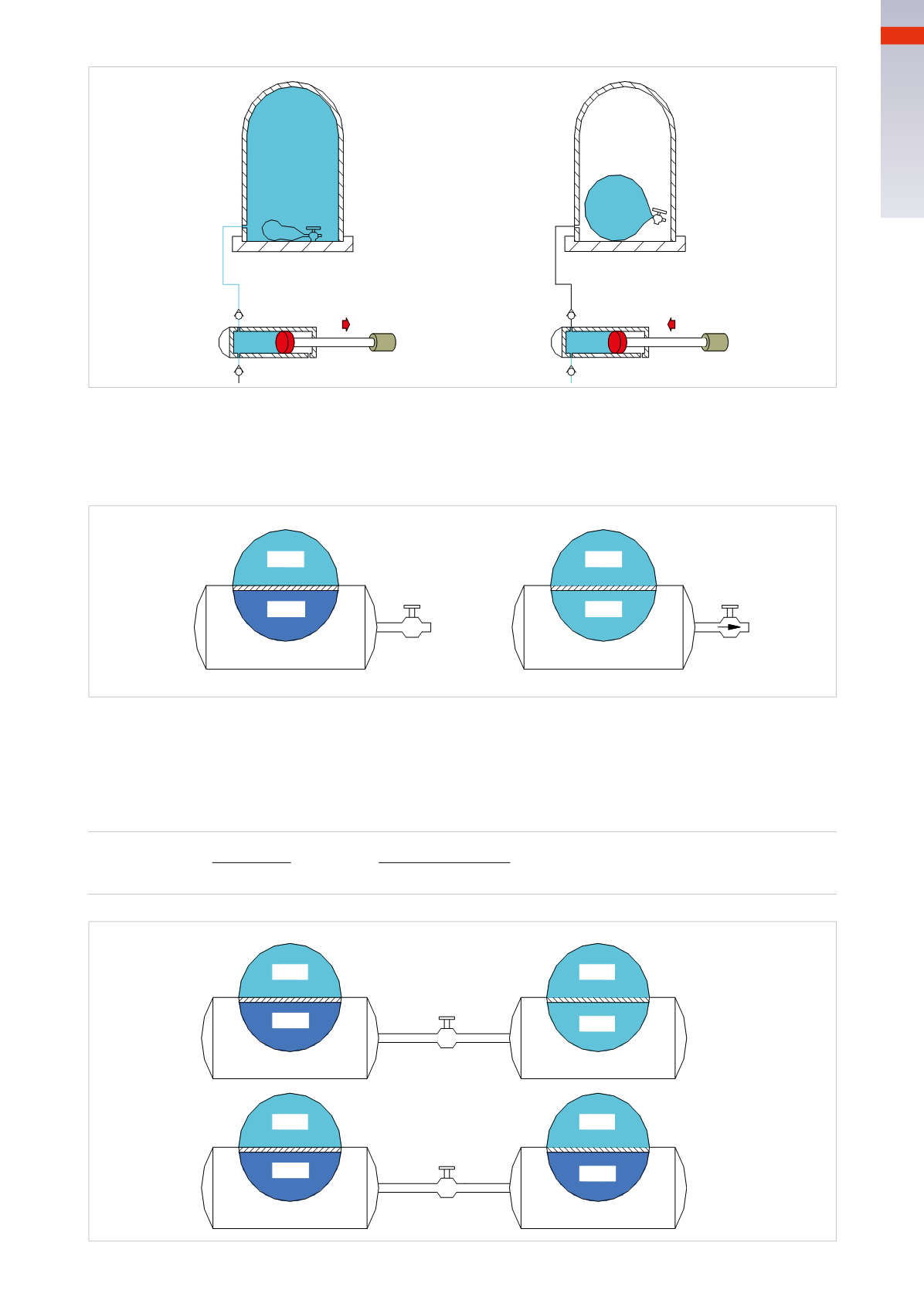
Figure 5
Two tanks, with pressure
p
1
= 5
bar
and
p
2
=1
bar
, are connectedwith a tap.
As inFig. 4, the opening of the faucet creates amovement of air from tank1 to tank2, i.e. from the higher pressure
to the lower, until the value of these two stabilizes at the equilibrium value
p
e
whichwe calculate from the average
of the two pressures:
p
e
=
p
1
+ p
2
5
[bar]
+1
[bar]
=3
p
e
=
3 bar
2
2
1bar
3 bar
1bar
5 bar
closed
open
1bar
3 bar
1 bar
1 bar
Fig. 5
1
15
CAMOZZI
>
PHYSICS

















