
The effect of temperature on gases
All bodies, regardless of their state (solid, liquid, gas), undergo changes in volumewhen subjected to a temperature
change. This phenomenon possesses different characteristics in the case of gases, which will take on the shape
of the container that contains them. Containers of different dimensions can be “full” with an equal volume of gas,
that is, the same amount of molecules. At constant temperature there is a fixed relationship between the volume
of the container and the amount of gasmolecules contained in it, i.e. between volume and pressure.
Therefore a variation in temperature will produce an effect on both the volume and the pressure, as we can see
from the figures below.
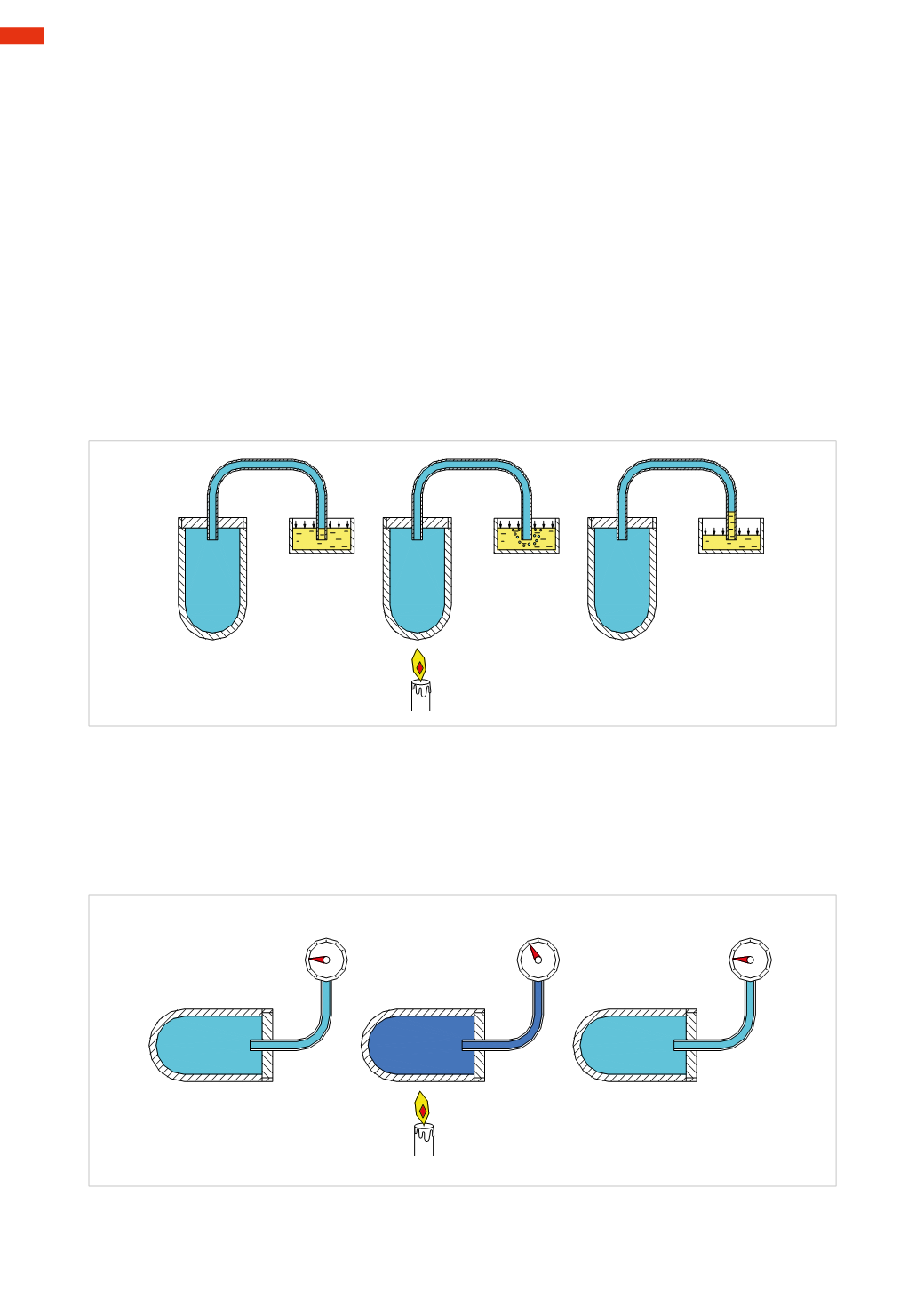
Figure 9
Heating at constant pressure, the gas increases its volume
A closed container full of air is connected by a pipe to a basin containing water. At an ambient temperature, the
air pressure inside the container is equal to the atmospheric pressure acting on the surface of the water in the
container. (Air will not escape from the tube andwater cannot enter it).
By heating the air in the container we can observe the bubblingwater, when turning off the flame; we can observe
the lowering of the water from its initial level and its ascent into the tube. The water bubbles, as the air heats
up, need to occupy a larger volume and the bubblingwater indicates the air molecules are exiting the tank. Once
there is no heat source, the gas reduces in volume and the water, pushed by external pressure, enters the tube,
occupying the volume that was previously occupied by the airmolecules dispersed into the atmosphere during the
heating phase.
Fig. 9
Figure 10
Heating at constant volume: the gas increases its pressure
The same container, filled with air at an absolute pressure
p
= 2
bar,
is connected to a pressure gauge, which
indicates a relative pressure of 1
bar
. This corresponds to the difference between the absolute pressure of 2
bar
,
inside the vessel and the atmospheric pressure of 1
bar
. By warming the air in the container, the air expands but
cannot escape, therefore its volume remains unchanged so it increases inpressure, as indicatedby themanometer.
As the air cools, the pressure returns to its original value, as indicated by the pressure gauge.
Fig. 10
When a gas is heated and has the ability to expand its volume, the pressure does not change, conversely when a
gas is heated but does not have the ability to expand, it undergoes an increase in pressure.
1
18
CAMOZZI
>
PHYSICS

















