
PHYSICS
0
20
40
313K
V
1
V
2
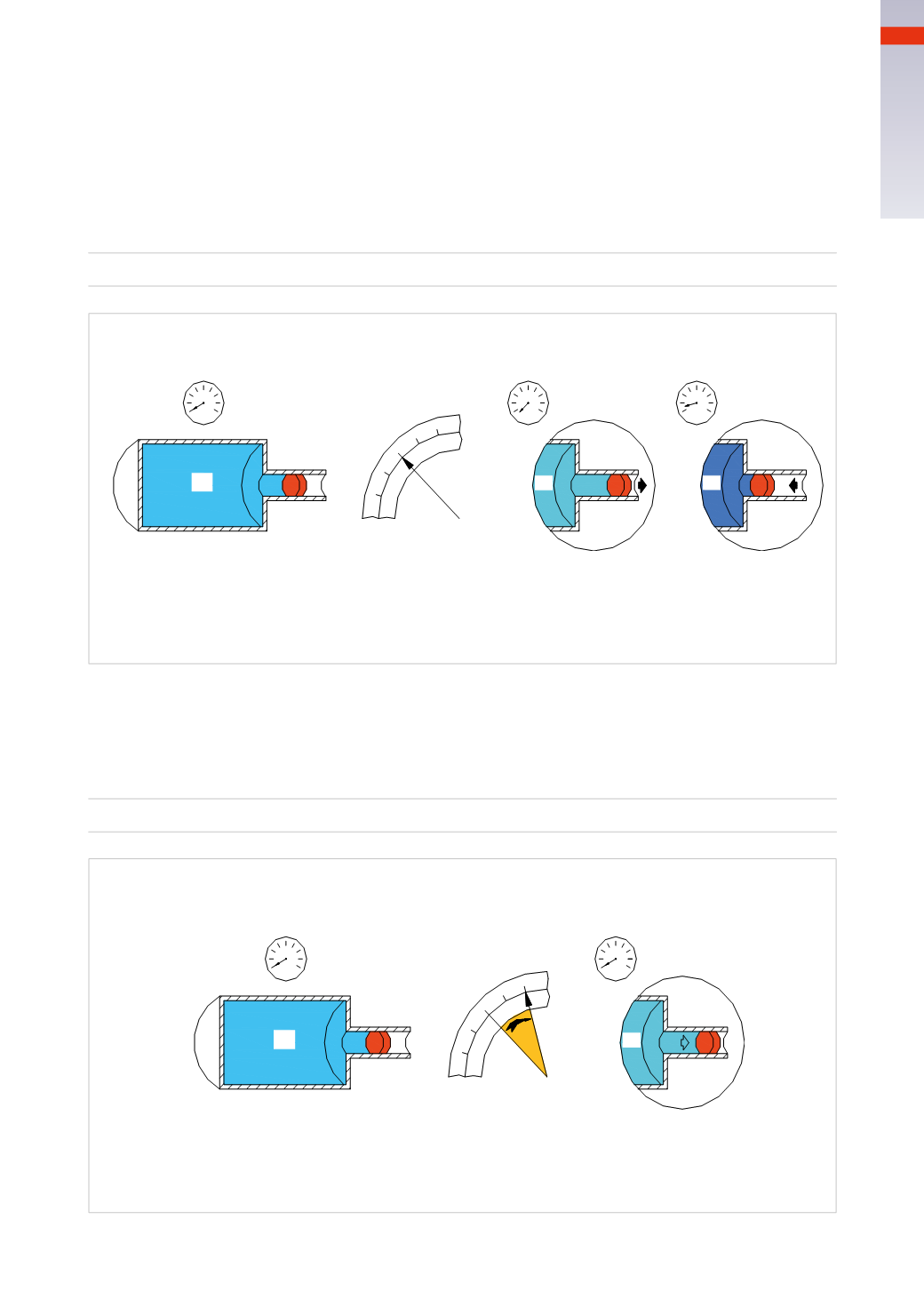
Figure 11
A tankwith volume
V
1
consists of two cylinders of different diameters, a piston of negligibleweight separates the
absolute pressure (inside the tank) and the atmospheric pressure.We assume that the pressure gauge is set at 0.
The room temperature i.e.
T
= 293
K
(20
°C
).
a)Maintaining a constant temperature
T.
If the piston is pulled outwards the volume inside the container increases,
the pressure gauge decreases falling below the value of zero, because the pressure inside of
V
2
is less than
atmospheric pressure.
b) If a Force is appliedwhich pushes the piston inwards, the volume of the tankwill decreasewhile the indicator
of the pressure gaugewill increase because the pressure inside of
V
2
is greater than atmospheric pressure.
V
1
:
V
2
=
P
2
:
P
1
0
20
40
293K
V
1
V
2
V
2
a
b
Fig. 11
Figure 12
Assume that we keep the temperature constant, and we act with an external Force on the piston, we raise the
ambient temperature of 20
°C
bringing it to a value
T
=313
K
.
Themomentum of themolecules, following their expansion, moves the piston outward until the volume increase
has compensated for the higher pressure.
V
1
:
V
2
=
T
1
:
T
2
Fig. 12
1
21
CAMOZZI
>
PHYSICS

















