
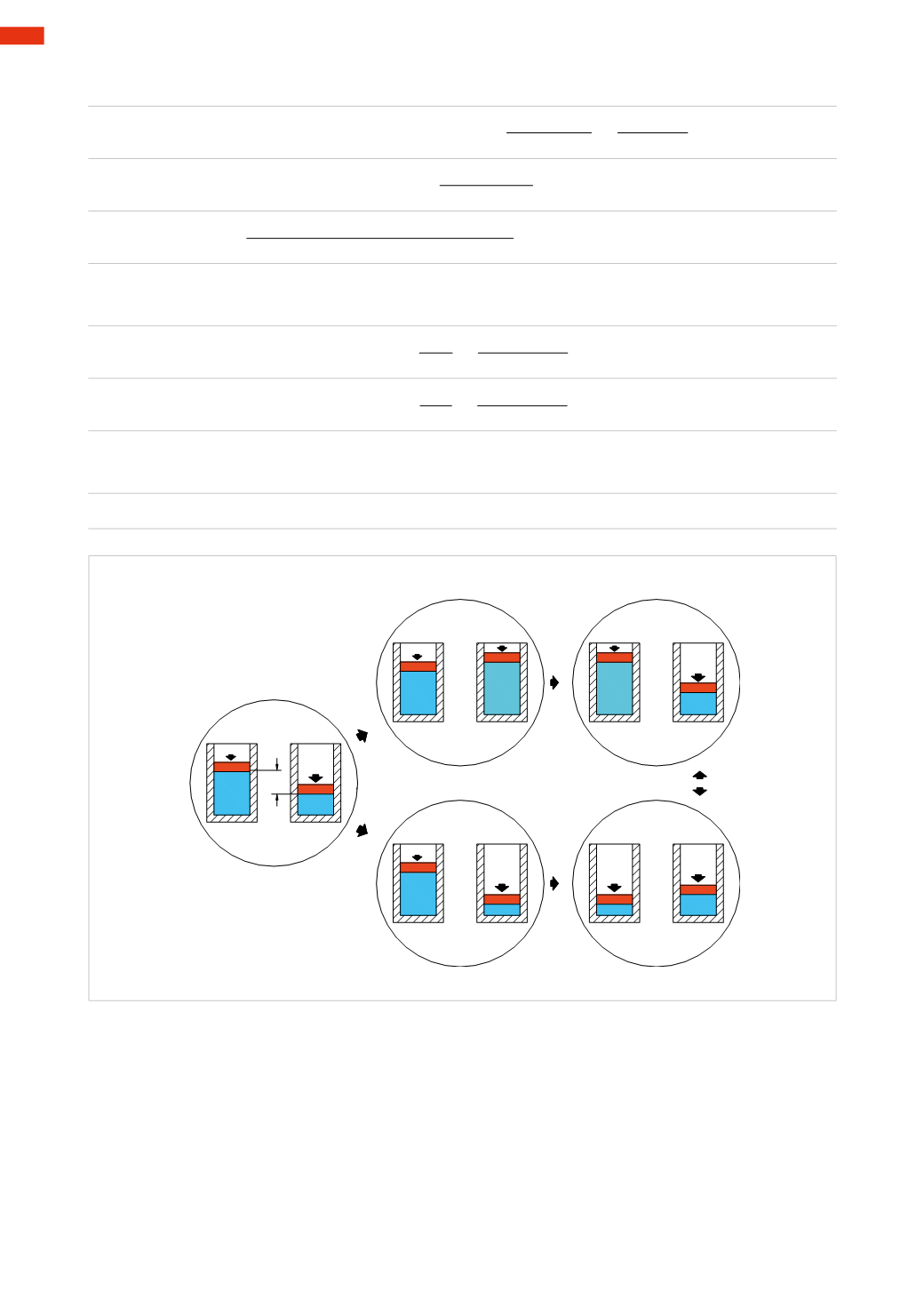
Another method that implements the two laws of Gay-Lussac
(p
1
* V
1
)
:
t
1
=
(p
2
*
V
3
)
:
t
2
p
1
* V
1
=
p
2
* V
3
t
1
t
2
V
3
=
p
1
* V
1
* t
2
p
2
* t
1
V
3
=
50
[N ⁄ cm
2
]
* 0,98
[dm
3
]
* 323
[K]
V
3
=
0,54
dm
3
100
[N ⁄ cm
2
] *
293
[K]
With the formula calculating the volume, it is possible to derive the change in height of the piston.
V
1
=
S
*
h
1
h
1
=
V
1
=
980
[cm
3
]
h
1
=
50
cm
S
19,6
[cm
2
]
V
3
=
S
*
h
2
h
2
=
V
3
=
540
[cm
3
]
h
2
=
27,5
cm
S
19,6
[cm
2
]
The piston is lowered by:
h
1
– h
2
=
50
[cm]
– 27,5
[cm]
=
22,5
cm
?
20 °C
50 °C
Problem
293K
323K
1
st
phase
323K
2
nd
phase
323K
293K
293K
1
st
phase
323K
2
nd
phase
293K
caseA
caseB
Fig. 14
1
24
CAMOZZI
>
PHYSICS

















